So, second in my run of posts targeted at A-level Chemistry and I’m touching on Aromatic Chemistry! What is it?? Essentially, it is the study of benzene and benzene type organic compounds. The word is referring to the very strange way the bonding occurs inside a benzene ring. This bonding is a little odd, and the oddness of this bonding was only recently discovered in the 1960s when they noticed something confusing about the bond lengths of inside the ring. But, more of that later. Let us start with the Benzene ring for now
The Kekule structure of Benzene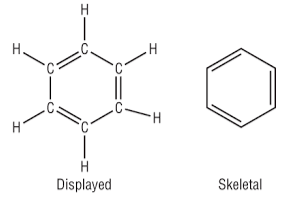
So, this is how we once thought Benzene looked. It would be called cyclohexa-tri-ene. We knew it was a ring of 6 carbon atoms with a hydrogen on each one, so this is how we theorised it must be. This structure is called the Kekule structure of Benzene, after August Kekulé. The model is wrong, and you’ll see why soon, but you can see why we used to think this.
Each carbon has 4 bonds, each carbon has one hydrogen. Everything seems fine, but even here there is something a little strange… The double bonds could, in theory, actually be in two different positions, and why be in one, rather than the other? See below for a diagrammatic explanation:
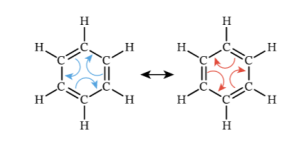
Incidentally, the name of the type of chemistry which looks at chains of atoms, as in most of organic chemistry, is called aliphatic, in case you start to see that word anywhere when learning about this.
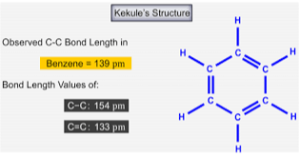
Bond lengths
The bond lengths of C-C bond and of a C=C bond are different. C=C bonds are shorter, but when measuring the bond lengths of benzene, they were found to all be the same length. This is our first big clue that the Kekule structure is incorrect. If the Kekule structure was correct, we should have seen two distinct bond lengths within the ring.
Hydrogenation of Benzene
The final nail in the coffin for the Kekule structure is the hydrogenation of Benzene. Hopefully, you know that hydrogenation simply means to add more hydrogen to a compound. We will imagine this happening with the Kekule structure. If we break all of the double bonds, each carbon atom will be able to hold another hydrogen atom, and so produce cyclohexane. We could do partial hydrogenation, and just break one double bond, but let’s do full hydrogenation for simplicity.
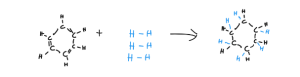
It is important to remember that, when we do this, we break bonds, which requires energy, and then make bonds, which gives out energy. You can think of breaking bonds as an endothermic process and making bonds as an exothermic process. So if it requires more energy to break the bonds, than the energy released to make the bonds, the reaction will be endothermic overall and vice versa for exothermic reactions. Doing a bit of maths, we find that the enthalpy change of adding one molecule of hydrogen to our Kekule structure of Benzene should be -120kJmol-1, and so for 3 molecules as shown above, it should be -360kJmol-1. A negative enthalpy change means an exothermic reaction.
But…
When we measure the enthalpy of the hydrogenation of benzene, it is actually -208kJmol-1. Now, importantly, this tells us two things. Firstly, the structure of benzene needs to be remodelled. Secondly, the actual structure of benzene as more stable than the Kekule structure of benzene, which of course is why it is that structure. Being given the choice, nature would always pick the most stable structure. Why does this finding mean that? Because the reaction is less exothermic. Meaning Benzene is more stable than we thought it was. It must have required more energy to break the bonds within Benzene to then add the hydrogens to make cyclohexane.
CHEMICAL BONDING AND ELECTRONEGATIVITY
So, what is the structure of benzene??
So, it is not single bond, double bond, single bond, double bond all the way around. If we first start off making everything a single bond then each carbon has a free (delocalised) electron which could bond in either direction around the ring. I’ve shown these electrons as dots on the diagram below:
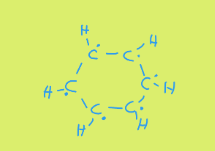
These delocalised electrons form a ring. Actually, they form two rings, one above and one below. See below:
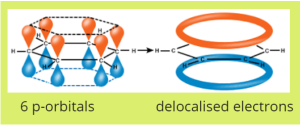
Another go at our first question
So aromatic chemistry is really referring to the study of molecules with this delocalised ring of electrons. Benzene is the most simple molecule with this ring, but aromatic chemistry includes other molecules which are derivatives of benzene and as such still contain the delocalised ring of electrons.
Ok. I hope that cleared up some understanding and I may return to this topic in future posts. Thanks for reading!
A bit about the author, Paul H:
 Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.
Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.