A recap
At GCSE we learned that the three types of chemical bonds:
- Covalent bonding: The electrons are shared between the atoms. The positively charged nuclei of each atom are attracted to the
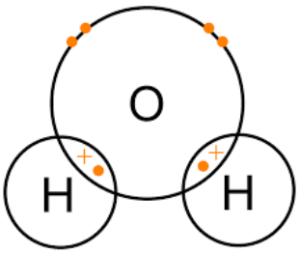 shared electrons. Covalent bonding usually lead to molecules being formed, which are groups of atoms bonded together in a particular way. Molecules are attracted to each other, but not so much, compared with the attraction between atoms within each molecule. However, there are a few exceptions in which covalent bonds lead to a giant covalent structure, such as graphite, diamond or silicon dioxide. In these cases, the group of atoms is never complete as such, as there are always atoms which have spare bonds and as such would happily bond with another atom, if it were to come along.
shared electrons. Covalent bonding usually lead to molecules being formed, which are groups of atoms bonded together in a particular way. Molecules are attracted to each other, but not so much, compared with the attraction between atoms within each molecule. However, there are a few exceptions in which covalent bonds lead to a giant covalent structure, such as graphite, diamond or silicon dioxide. In these cases, the group of atoms is never complete as such, as there are always atoms which have spare bonds and as such would happily bond with another atom, if it were to come along.
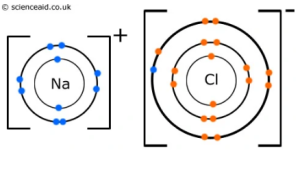
2. Ionic bonding: One atom donates electrons to another. This causes one atom to be positively charged and the other negatively charged. As a result, the two ions are attracted to one another. The ions form an ionic lattice, which like a giant covalent structure, has no size limit. No matter how large the lattice is, there is always room for more ions to join on to it.
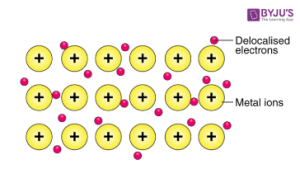
3. Metallic bonding: When metal atoms join together, the outer electrons are free or delocalised and form what is called a referred to as a sea of electrons. This ‘sea’ is of course negatively charged and the positively charged metallic ions are attracted to it, which is what forms the bond.
Introducing electronegativity
At A-level we learn that the difference between ionic and covalent bonds is actually not all that clear cut. There’s covalent, ionic and er… sort of covalent! This last category is more commonly referred to as polar bonds.
So, what is electronegativity? Essentially, it is how much an atom attracts the bonding pair of electrons within the covalent bond. At GCSE we tend to think of the atoms being shared equally, but that is often not the case. For example, a molecule of Hydrogen Fluoride the bonding pair of electrons are much more attracted to the Fluorine atom and as such you are more likely to find them there, than around the hydrogen atom.
Below is what looks like the periodic table, but the numbers you see are referring to the electronegativity of the element. The higher the number, the more attractive bonding electrons will find that atom. These numbers are determined using the Pauling scale of electronegativity invented by Linus Pauling.

WHAT IS A MOLE? A 3 part series
Trends in electronegativity
You will notice a couple things in this table:
- Some noble gases do not seem to have any electronegativity.
- If you stay within the same period (row) the electronegativity increases from left to right, apart from some noble gases.
- If you stay within the same group (column), the electronegativity increases as you go upwards.
- The most electronegative element is fluorine, in the top right (excluding noble gases)
- The least electronegative elements we can see are in the bottom left: Caesium and Francium.
Let us try to understand these observations:
Observation 1: Noble gases have a full valence shell of electrons and as such do not have any need for attracting electrons. So, what about krypton and Xenon?? It was discovered in the 1960s that these two elements can react with very electronegative elements such as fluorine and oxygen.
Observation 2 to 5: The rest of the observations can be explained together and it helps here to think of how we understood reactivity at GCSE. As you go down a group, you are adding another shell of electrons. This means that bonding electrons are further away from the positively charged nucleus and as such will not feel as much of an attractive force. As you move across the period, you are adding extra protons to the nucleus. This means that the nucleus will become more positively charged and so would attract the bonding electrons more strongly. So, the element with the most positively charged nucleus but with still a small number of shells is fluorine; whilst the opposite is true for Caesium and Francium.
Polar bonds
Let’s take the compound Hydrogen Chloride: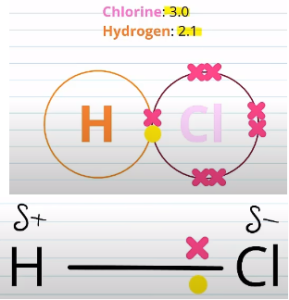
Chlorine has a higher electronegativity than hydrogen and so the electrons are going to be more strongly attracted to the chlorine than the hydrogen. As such, the electrons will be closer to the chlorine than the hydrogen. This can be shown in a second diagram on the right.
As a result the chlorine will be slightly more negatively charged than the hydrogen and this charge difference is called a dipole. This is represented by the small delta positive and negative symbols on the diagram. This slight charge difference is always present and so we can call it a permanent dipole. There are such things as temporary dipoles which you will come across during your A-level.
So, there is more to learn along this topic, but I hope for now this should suffice as a brief introduction to electronegativity
A bit about the author, Paul H:
 Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.
Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.


