This is quite a mathematical area of the Chemistry A-level, but we can start off with a brief recap of some fundamental principles within Chemistry, which are not really mathematical
The Arrhenius equation (above) relates to the rate of reaction and contains the following variables within the equation:
k = the chemical reaction rate (variable units)
A = the pre exponential factor (same units as k)
Ea = the activation energy (J mol-1)
R = the gas constant (J K-1 mol-1
T = temperature (K)
How the rate of reaction might change?
Let’s think about how these variables should impact the rate of reaction.
Temperature
It stands to reason that a higher temperature should lead to a higher rate of reaction. The molecules are moving more quickly and so there will be more collisions per second and each collusion is more likely to be successful. So presumably, the equation above should show that…
…a higher temperature leads to a higher rate of reaction.
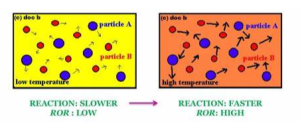
Activation energy
Next is activation energy. Remember that the activation energy is the energy required for the reaction to occur. If the activation energy is low, then the collisions are more likely to be successful. So the equation above should show that…
…a higher activation energy leads to a lower rate of reaction.
And now the maths…
So let’s see if the equation does what it is supposed to do.

Let’s rewrite the equation as…
k = A e-x
Where ![]()
Hopefully you can see that A is directly proportional to k from the top equation.
We know from our GCSE Maths lessons that…
a-b = 1/ab
So,
e-x = 1/ex
If x is large, then ex would be large. This would make e-x small.
So, A large x value means a small k value and a small k value means a low rate of reaction. In other words, a large x value means a low rate of reaction.

As you can see, x is directly proportional to the activation energy, so a large activation energy, means a large x value, which would mean a low rate of reaction.
We can also see that x is inversely proportional to the temperature. So, a high temperature would mean a low x value, which would mean a high rate of reaction.
So, it all checks out. The equation does what it is supposed to do. Awesome. Truly awesome.
CHEMICAL BONDING AND ELECTRONEGATIVITY
The other Arrhenius equation…
You may have come across this other equation which looks similar, but not the same. To understand where this came from, we need to understand a very useful mathematical tool called logarithms, or log for short.

What are logs?
So, logs are the final piece of the jigsaw puzzle when it comes to an equation like the one below:
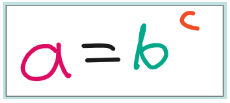
If we want to rearrange the equation to make b the subject, we use the mathematical operation known as a root:

But how we can we make c the subject? How can find the power in the first equation if we knew a and b? The answer is use logs! In bc, we would say c is the power and b is the base. So when we use a log, we need to include in it what base to use.
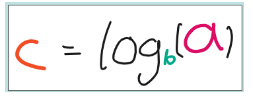
On your calculator, you have a few log buttons that can help you with your Arrhenius equation:

In the blue circle at the top, you have a log button where you can choose the base.
Eg. Log2(8) = 3, because 23=8. Remember, logs help you to find the power.
In the red circle on the left, you have a log button which does not seem to have a base. It does, but is often not written. The base is 10.
Eg. Log (10,000) = 4 , because 104 = 10,000.
In the green circle on the right, you have ln. (Lower case L, not capital I) This is often pronounced “Lun”.
This last one means natural log it is a log with the base e. e represents a mathematical constant called Euler’s number. We won’t worry much about the nature of this constant, but if:
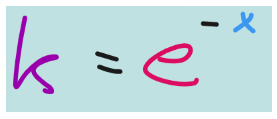
Then,
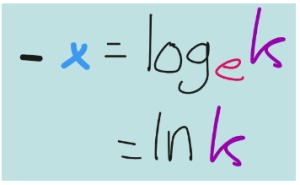
LEARNING SCIENCE – CAN IT MAKE YOU HAPPY?
Deriving the other Arrhenius equation
So, we can take the equation:

And natural log both sides of the equation:

In logarithms, there are some log rules. See below:
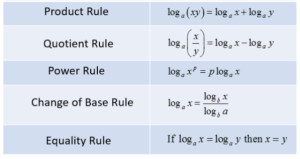
I’m going to apply the product rule to our equation so far:
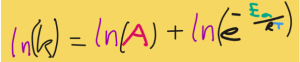
On the second term, the natural log cancels out the e, so now we have:

And finally, with a little bit of moving terms around we have…

If you have followed me up to this point, and you have only just today heard of logs, well done. If you have been learning this in A-level maths then some of that might have been fairly simple, but if you are struggling with logs in general I shall be writing a post on that topic at some point in the future.
I hope that was useful and you now understand the Arrhenius equation a bit better. I’ll see you again in the next post! 🙂
A bit about the author, Paul H:
 Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.
Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.