An introduction to heat and temperature
Let’s try and get our head around heat and temperature. A concept we thought at least this one we understood and then our Physics teacher took that away from us as well didn’t they??
7 WAYS TO GET THE BEST RESULTS FROM PRIVATE TUTORING
What is temperature
Some time ago we started to notice that things changed when it got hot or cold. Specifically, we noticed that matter (a sciency word for ‘stuff’) got bigger, or expanded, when it was hot and got smaller, or contracted when it was cold. Some clever scientists used this idea to invent various thermometers and then marked numbers on it so that we could actually measure how hot or cold something is. You know us humans always like measuring…
Now, we may not have understood how these thermometers did what they did, and I’m sure many of you never gave it much thought. If it’s hot or cold, the thermometer says so. But, how does it do that? If I search ‘thermometer’ on the internet and choose ‘images’, I mostly get images like the one you see here. These are not the only thermometers, but we will stick to this design for now. The first thermometer actually used air instead which expanded in heat and contracted in cold, but it was not as accurate as liquid and so thermometers started to use liquids soon after and we stuck to that.
The particle model of matter
When it gets hotter, the liquid expands inside the thin glass tube and so rises up. But why?
The basic reason is that the particles in the liquid move faster and move greater differences because they now have more energy. This causes them to spread out a little bit. This can be a little confusing because we learn pretty early on that liquids cannot be compressed and have a constant volume, but this is only at a constant temperature.
The above paragraph only makes sense if you are happy with the idea that matter is made out of particles. We have all seen the diagram below many times by now I’m sure, but we never actually see these particles our science teachers assure us are around do we? Well, they do exist, I am quite sure. This idea of matter being made out of particles is known as the particle model of matter.

A new definition of temperature
So now we have an understanding of how our thermometer works, we could say that temperature is a measure of how fast the particles are moving, or even a measure of the average kinetic energy of the particles. If you give the particles energy they move more quickly and so take up more space, which is why the liquid in a thermometer rises! Here is a simulation of a thermometer which you can play with and see the particles in it moving around.
So, if temperature is how quickly things are moving, at zero temperature they should… erm… not be moving?? But that can’t be right, I’ve walked around at 0°C and was able to breathe the air and survive. If particles had stopped moving, the air should have not been in a gaseous form, it should have been solid and the particles should not have even been vibrating! And what the heck would negative temperatures be? Well… Not all temperature scales start at the same place.
We chose to put 0°C at the freezing point of water and 100°C at the boiling point of water which is useful for many purposes, but is not exactly correct when we think of our new definition of temperature is it? We need a new scale!
Enter Kelvin!
Here he is. Looking rather sleepy in this photo, but he’s no doubt been hard at work coming up with a new scale for temperature! What do we call this new scale? The Kelvin scale!
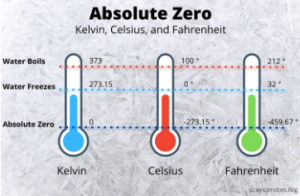
What happens at zero kelvin? Nothing! Nothing moves. Particles in a solid do not even vibrate! We call this point absolute zero but… It turns out that it is impossible to actually reach this point.
That shouldn’t stop us from defining it though. It is impossible to get anything to actually sit still for a moment! I know that feeling…
Ok, so is heat the same as temperature??
Erm… Not really. If you think of how the two words are used, heat is something which something has or something which we can give to something. We do say ‘heat up’ as well, but that really means the same thing as to give something more heat so as it now has more heat in it.
Temperature, on the other hand, is a measure of how hot or cold it is. So, when we give something ‘heat’, what are we actually giving? Well… energy, right? So, heat is the energy we give it and temperature is the measure of average kinetic energy of the particles. Now it’s sounding like the same thing again! Not exactly… The keyword here is average. I mean the average kinetic energy of each particle.
However, if we give something energy, it should get hotter. Pretty straight forward right? However, if you plot a graph of heat against temperature something strange happens. I will have to leave that for another blog post though I’m afraid!
PHYSICS PRACTICE QUESTIONS – WHY ARE THEY SO ESSENTIAL?
A bit about the author, Paul H:
 Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.
Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.