Intermolecular forces 1: Van der Waals
So, in this series I want to go over what intermolecular forces are, the three types to consider and what sets each type apart from the other.
Firstly, what do we mean by intermolecular forces? The inter part essentially means between, as in international travel means travel between nations. So, intermolecular forces means forces between molecules, not forces within molecules. That is quite important. Forces within molecules, in other words The regular kind bonding such as covalent, ionic and metallic, is referred to as intramolecular forces. We are looking at the forces between one molecule and another nearby molecule. These forces are much weaker than the bonds within a molecule.
The three types of intermolecular forces
So, the three types we shall look at are:
- Van der Waals (often referred to as vdw)
- Dipole-dipole
- Hydrogen bonding.
First thing to note here is that I have arranged them in order of strength, with the weakest being at the top with Van der Waals, going down to the strongest at the bottom with hydrogen bonding.
THOUGHTS THAT CAN STOP YOU STUDYING
The weakest of the three: Van der Waals
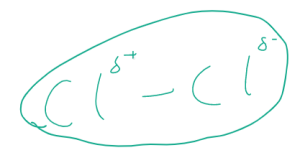
Let’s imagine a molecule, for example chlorine, Cl2. The two atoms are bonded by a single covalent bond, but the electrons are constantly moving around and at any one time, they could be more around one atom than the other. We could say that the electron density is higher around one atom than the other. The diagram below shows the electron density is higher around the right hand atom within the molecule.

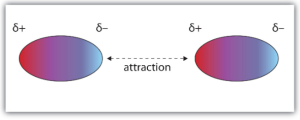
As a result, the part of the molecule that has a lower electron density will have a slightly positive charge. The other side will have a slightly positive charge.
We refer to this mis-distribution of charge as a dipole. In this case, it is a temporary, or induced dipole because no sooner do we have an arrangement like the one above, the distribution could change and so the dipole would reverse. Of course, there would be moments of no dipole and the strength of the dipole will fluctuate also.
So, two neighbouring chlorine molecules may feel a slight attraction to each other as the slightly positive area of one molecule is attracted to the slightly negative area of another.
Something which is often not explained here, is that a randomly occurring dipole in one molecule causes a dipole in the other molecule, hence the expression induced dipole. If a positive charge from the first molecule approaches the electron cloud of the second molecule it causes the electrons in the second molecule to be attracted to it which causes that part to be negatively charged. The opposite happens if the first molecule’s negative side approaches the second molecule. A similar thing occurs when you charge a balloon by rubbing it on your head and it sticks to a neutral wall. Click here for a simulation of this.
RADIOACTIVITY, NUCLEAR DECAY, RADIATION… WHAT’S THE DIFFERENCE?
How molecular size affects things
It must be no surprise that molecules with more atoms in them would have stronger van der Waals forces, as they have more surface area. However, less obvious is that molecules with larger atoms in them also have a stronger van der Waals. This is because more electrons are able to move around the molecule and lead to a stronger dipole
Saturated and unsaturated fats
Another interesting case study is saturated and unsaturated fats. Saturated fats include most animal fats and occur in butter and lard. They also occur in coconut oil You may have noticed that they are always solid at room temperature. Whereas unsaturated fats, such as olive oil and other vegetable oils are liquid at room temperature. This must be because room temperature does not produce enough energy to melt saturated fats, but does to melt unsaturated fats. Why is this?
Unsaturated fats, due to the double bonds, tend to have kinks in them and not lie flat next to each other, whereas saturated fats are more straight which means they can lie very close to one another. Because of this, the saturated fats get closer and can produce larger van der Waals forces. See the diagram below.
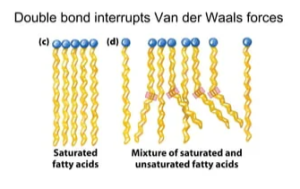
As you can see, the double bond bends the molecule and they are less able to lie close together.
Summary
So, I have gone over the first of the three intermolecular forces, which is also the weakest. The next one is usually called dipole to dipole, but more specifically involves permanent dipoles. In van der Waals, it is technically a dipole to dipole also, but in this case they are randomly occurring dipoles, or more accurately: One randomly occurring dipole inducing a dipole in another molecule leading to an attractive force.
I hope that cleared up a little bit of confusion on the topic. I shall see you in the next post!
A bit about the author, Paul H:
 Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.
Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE. He also teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.


