Completing a titration calculation
This is the third post in my series on moles. This one covers performing a titration calculation. You can click here to see part one and part two.
In this post, we look at how to use a titration result to calculate the concentration of an acid or alkali, as in this question:

25.0 cm3 of 0.200 mol/dm3 sodium hydroxide solution reacted with 28.7 cm3 sulphuric acid. Calculate the concentration of the sulphuric acid in mol/dm3 .
2 NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O(l)
What is a titration?
That’s the first question we need to answer.
Basically, it is a neutralisation reaction used to identify the concentration of an acid or alkali. We carefully drip one of the reactants through a burette into another which is classically held inside a conical flask. Using universal indicators and constantly stirring we note from the burette the volume required to neutralise the acid or alkali.
So we have substance A of known concentration and known volume and substance B will have an unknown concentration but a known volume by the end of the experiment. We can refer to substance A as the ‘known’ substance and substance B is the ‘target’ substance, as that is the substance we are trying to find out about.
A note about concentrations, volumes and moles

If the concentration of something is 0.200 mol/dm3 of sodium hydroxide, that would mean if we had 1dm3 of the solution then inside the solution would be 0.2 moles. So if we had 2dm3 then we’d have 0.4 moles. Using this thought process and understanding we can see the formula that relates moles, concentration and volume is as follows:
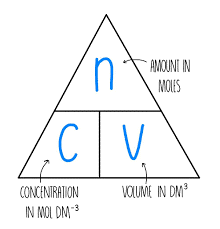
Number of Moles = concentration X volume
This could then be put into a formula triangle:
A dm3 seems an odd unit of volume, especially when the volumes we’ll most likely be measuring will be in cm3. Without going into too much detail I’ll just say that:
-
- 1cm3 = 1ml and 1000cm3 = 1dm3
- Also, 1000ml = 1 litre.
- So, 1dm3 = 1 litre.
Solving the problem

Let’s return to the problem and solve it now with our improved understanding.
25.0 cm3 of 0.200 mol/dm3 sodium hydroxide solution reacted with 28.7 cm3 sulphuric acid. Calculate the concentration of sulphuric acid in mol/dm3.
2 NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O(l)
Step 1 – identify the known and target substance
We know the concentration and volume of the sodium hydroxide so that is our known substance. The target substance is the sulphuric acid. We are to find out the concentration of this. I’ve colour coded this to help follow along.
Step 2 – Convert the volume(s) into dm3
The volumes will usually be in cm3, but the concentrations are in mol/dm3, so in order for us to use the formula I introduced earlier we need them in consistent units. There are 1000cm3 in 1dm3, so to convert cm3 to dm3 you need to divide the volumes by 1000.
Volume of sodium hydroxide
25.0cm3 = 0.025dm3
Volume of sulphuric acid
28.7 cm3 = 0.0287dm3
Step 3 – calculate the number of moles of known substance
Being as we know the concentration and volume we can use the formula triangle from earlier to calculate the moles.
Moles of sodium hydroxide
= concentration X volume
= 0.200 mol/dm3 X 0.025dm3
= 0.005 moles
Step 4 – Use the balanced equation to work out the number of moles of the target substance
We can see from the equation that if we had 2 moles of sodium hydroxide, we could react it with 1 mole of sulphuric acid. So it’s a 2:1 ratio. We have 0.005 moles of sodium hydroxide so it could react with 0.0025 moles of sulphuric acid.
Step 5 – calculate the concentration of the target substance
We know that 0.0287dm3 of sulphuric acid was needed to complete the titration and so within that volume there must be 0.0025 moles of sulphuric acid. By using the formula triangle from earlier again:
Concentration of sulphuric acid = Moles ÷ volume
= 0.0025 moles ÷ 0.0287dm3
= 0.0871 mol/dm3 (3sf)
So, there you have it. Now, questions may vary slightly in that they may want the volume instead of the concentration, but in that case, they would give the concentration or some other means of getting it.
Notice also that with titrations there is no need to consult the periodic table to find the atomic mass or anything. However that does not mean that a sneaky follow-up question asking you to convert the moles into mass may not be included, but that is unusual.
That concludes the 3 part series on moles. The next post shall be a little different and then I may do another couple of guides on another commonly misunderstood topic within science.
Thanks for reading and I shall see you in the next post!
A bit about the author, Paul H:
 Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE, plus teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.
Paul is a qualified and experienced Physics, Maths, and Science teacher, now working as a full-time tutor, providing online tuition using a variety of hi-tech resources to provide engaging and interesting lessons. He covers Physics, Chemistry, Biology, and Science from Prep and Key Stage 3 through to GCSE and IGCSE, plus teaches Physics, Maths, and Chemistry to A-Level across all the major Exam Boards.